Whoever thinks of truffles usually has a culinary delicacy in mind. Wild boars are also truffle lovers, but they love other species than their human counterparts: deer truffles, which, however, are not real truffles at all. Their fruiting bodies represent only a convergent development among the ascomycetes (tubular fungi). The Deer Truffle, takes its name from the Greek, Elapho- (deer) and -Myces (fungus).
Elaphomyces (“Deer truffles” or “False truffles”) is one of the most important ectomycorrhizal fungal genera in temperate and subarctic forest ecosystems. But it is also one of the least documented in public databases. The current systematics is based mainly on macromorphology and does not differ significantly from that proposed by Vittadini (1831). Within the 49 globally recognized species, 26 were originally described from Europe and 17 of them before the 20th century. In addition, very recent phylogenetic treatises of the genus are based mainly on some non-European species. The most common European species are still poorly documented.
First of all: for humans deer truffles are inedible or not tasty, especially older fruiting bodies are not edible. Since their fruiting bodies grow underground for this purpose, the commercial interest in the hypogeous genus Elaphomyces is very low. Accordingly, little is known about the biology of these fungal species.
There also seems to be no interest in deer truffles from the scientific community so far. This is surprising, because Elaphomyces are important mycorrhizal fungi for spruce and pine and beech, which gives them a corresponding importance. It is probably the most abundant and widespread of all truffles in the world, fruiting nearly the whole year.
Deer truffle – profile of False Truffles
Deer truffles are underground growing mushroom bodies, which when fully grown look like marzipan balls rolled in cinnamon. Latin name is Elaphomyces. spec. There are about 15 different species in Germany, the most widespread species is the Warty Deer Truffle, Elaphomyces granulatus. The second most common is the reticulated or small-bodied deer truffle Elaphomyces muricatus.
Fig. 2: Exposed deer truffle (Elaphomyces granulatus) in 8 cm soil depth ( border O horizon/A horizon). Sample plot B1
The mean depth at which the 82 collections of deer truffles were found was 5 cm, with truffles located between 1 cm and 16 cm soil depth, both in the humus layer and in the mineral horizon.
Deer truffles are mycorrhizal fungi that form symbiotic relationships with various tree species. However, the visible deer truffles are only the fruiting bodies of the fungal mycelium, which grows year-round. Unlike the fruiting bodies of other mushroom species that grow above ground only seasonally (especially in the fall), the fruiting bodies of deer truffles grow year-round.
The fruiting bodies consist of a relatively hard, rubbery bark and are filled with spores when mature. The rind usually has a wart-like texture and is light brown in color. The color and consistency of the spore material varies as the truffle matures. The initially light gray solid mass inside turns darker and darker as it matures. When fully ripe, the truffle is loose, powdery and deep purple inside.

Fig. 3: Deer truffle (Elaphomyces granulatus) on moss.
The truffle has a fine warty to granulus, light colored surface, 2-4 mm thick. At the ripe stage it contains a powdery black mass of spores (Fig. 8). The fruit bodies are round, light brown to rust-colored, 1 to 5 cm in size and have a mass of up to 18 g (but usually only a few grams).
Ecology
Deer truffles are mycorrhizal fungi, i.e. fungi that enter into a symbiosis with tree roots with the purpose of exchanging (nutrients) substances with one another. Elaphomyces species form an ectomycorrhiza with their host trees. This deer truffle ectomycorrhiza is a short-distance exploration type (Reess & Fisch 1887, Agerer 2001, Agerer et al. 2002). Therefore, mycelium and fruiting bodies can only be found in the immediate vicinity of the fine roots of the mycorrhizal tree. An occurrence outside the root radius of trees is probably not possible. However, it has been proven that Elaphomyces sp. can form fruiting bodies for one or more years even after the partner tree has been felled (Hesse 1889, Hawker 1954).
Deer truffles occur under mature or old trees (Reess & Fisch 1887, Hawker 1954, Szemere 1965, Luoma et al. 1991, North et al. 1997, North & Greenberg 1998, Luoma et al. 2003, Meyer & North 2005), the single specimens can also be in the midst of younger holdings (Szemere 1965). The management of the forest, especially clearings and clearcuts, have negative effects on the biomass and fruiting abundance of deer truffles. So far, this has only been proven in the USA (North et al. 1997, North & Greenberg 1998, Gomez et al. 2003, Luoma et al. 2003, Gomez et al. 2005). The occurrence, at least of E. granulatus, correlates positively with the deadwood mass of the forest concerned (Meyer & North 2005) as well as with a well-developed humus layer with many fine roots (North & Greenberg 1998). Here, too, it should be noted that the studies in question were carried out in the USA and do not have to apply to Europe. Deer truffles are rarely found under a heavily developed herbaceous layer (Hawker 1954); there are controversial statements about the influence of a thick layer of moss (Hawker 1954, Lange 1956). In general, Elaphomyces species prefer medium soil moisture; Waterlogging and very dry soil are not tolerated, the same applies to steep slopes (Hawker 1954, Szemere 1965, Luoma et al. 1991). Only in drier habitats is the fruiting body formation positively correlated with precipitation (Meyer & North 2005).
Species identification & phylogeny
Some species can be easily identified by their external morphology based on your peridium (in cross section) or your spores. In case of doubt, only genome determination by DNA extraction and DNA sequencing will help (ITS & PCR: Internal transcribed spacer (ITS) refers to spacer DNA).
Mycorrhizal partners
Elaphomyces granulatus is predominantly associated with spruce and pine, while E. muricatus has predominantly beech as tree partners.
Systematics
The genus Deer truffle belongs to the subdivision Tubular Fungi, class Eurotiomycetes, order Eurotiales and family Elaphomycetaceae:
Subdivision: true tubular fungi (Pezizomycotina).
Class: Eurotiomycetes
Subclass: Eurotiomycetidae
Order: Eurotiales
Family: Deer truffle relatives (Elaphomycetaceae)
Genus: Deer truffle
Importance of Elaphomyces spec.
Mycelium: Important mycorrhizal fungus for spruce and pine in Germany
Fruiting body – sporocarp: size up to a maximum of approx. 5 cm and 15 g weight
Important in the diet of wild boar and some small mammals such as the bank vole and dormouse (Schickmann et al. 2012, Cázares and Trappe 1994).
Fruiting bodies accumulate radioactivity (Cs-137), leading to Cs-137 contamination of wild pigs
Spread of spores: via mycophagy, i.e., animals. Untouched, the fruiting body slowly decays through peridial decomposition. The blue-black spores are then released as a compact “block” in the forest soil.
Peridia & Gleba
Figure 4 shows a cross-section of an Elaphomyces granulatus weighing about 7 g. The light peridium can be clearly distinguished from the dark gleba filled with spore mass. The fruiting body shell is very coarse and only a few mm thick.

Spores and dissemination
Mature deer truffles harbor a dark blue to purple spore mass inside that can be pulled apart as tough filaments.
Reynolds (2011) concludes in her dissertation on Elaphomyces that while it clearly relies on animal vectors for excavation and dispersal. However, the history of long-distance dispersal of the genus and current spore trajectories suggest that Elaphomyces spores can also be dispersed passively in the air. This is because the spores can remain airborne long enough to be dispersed over long distances by the wind.
Fruiting bodies
Fruiting bodies are formed at the boundary of foliage or humus layer and firmer soil (“hard pan”) at a depth of 5-15 cm (Hesse 1894, Hawker 1954, Lange 1956, De Vries 1971, Kreisel 1996). In our own studies, the mean depth of the fruiting bodies of 126 deer truffle collections was 5.5 cm, with a range of 1 cm to 16 cm soil depth (Fielitz 2005). Deer truffles are often pressed against stones or larger roots (Hawker 1954, Szemere 1965). At least fruiting bodies of E. granulatus are preferentially found within about 2 m of the trunk of the mycorrhizal tree (North & Greenberg 1998). Fruiting body formation is very uneven; in one study, 78% of the fruiting body mass of E. granulatus was located on 0.5% of the study area (Meyer & North 2005).
Fruit bodies are formed all year round (Reess & Fisch 1887, Fischer 1897, Hawker 1954, De Vries 1971, Luoma et al. 1991), the maximum fruit body formation is reached in the winter half-year (Fischer 1897, Jahn 1949, Luoma et al. 1991). However, it cannot be ruled out that these observations can be traced back in part to the increased burrowing activity of game in winter and the resulting easier detection of the fungi. The fruit bodies and also the spores are also only decomposed very slowly (Reess & Fisch 1887, Hawker 1954, North et al. 1997), and can still be detected for years.
Development
True truffles often have a distinctly seasonal development of fruiting bodies. Summer truffles are found in the summer months, while the harvest season for many other tuber species is in the fall and winter.
Fruiting bodies of deer truffles, on the other hand, are found all year round, in our experience even most frequently in spring. Usually different developmental stages are found side by side.
There is a lack of information in the literature on how long the fruiting body development lasts and whether seasonal cycles are present. Rees (1887) suspects several years. We assume a developmental time from youngest primordium to adult deer truffle with spore maturity of several months to a year.
No recent publications on the subject can be found on Google scholar. One ongoing project mentioned can be found under the title Radiation Protection Research – Program Report 2018 – DORIS (bfs.de). Contractor is umweltanalysen.com
Deer truffle as food for wild animals
Deer truffles are eaten by soil invertebrates (especially ants, beetle larvae, nematodes, annelid worms). These seem to prefer the peridia of the fruiting bodies, the spore mass is less often attacked (Hawker 1954, Szemere 1965, Reddell & Spain 1991). In addition to wild boars, vertebrates also include roe deer and red deer and fallow deer, badger, squirrels, mice and various American small mammals are known to be consumers of deer truffles (Reess & Fisch 1887, Rancken 1910, Hawker 1954, Szemere 1965, Maser et al. 1978, Grönwall & Pehrson 1984, Kotter & Farentinos 1984, Blaschke & Bäumler 1989, North et al. 1997, Luoma et al. 2003, Gomez et al. 2005). This diet is called mycophagy.
It is possible that the passage through the digestive tract of animals is necessary for spore germination of the deer truffle (Reess & Fisch 1887, Kotter & Farentinos 1984). In addition, the fruiting bodies serve as a substrate for core lobes (in Europe Cordyceps ophioglossiodes, C. capitata), with infestation the formation of spores does not occur (Szemere 1965, Benkert 1975, Helfer 1991, Sung et al. 2007). Core lobes are parasitic Ascomycetes that attack deer truffles and grow on them. Trees mycorrhized with E. granulatus do not have any mycorrhiza of Xerocomus badius; the species seem to be mutually exclusive (Agerer et al. 2002).
Distribution – Maps
The genus Elaphomyces has been found in the northern and southern hemisphere (Castellano & Bougher 1994). According to Paz et al. (2017), there are 49 Elaphomyces species worldwide of which 23 species occur in Europe. In many parts of the U.S., they are the most important hypogeous species in terms of biomass and frequency of occurrence. Due to the underground way of life of the fruiting bodies, the evidence of Elaphomyces is significantly lower than that of the above-ground fructifying fungi.
Germany – Deer truffle species in Germany
Presumably these Elaphomyces species occur in Germany
E. aculeatus
E. anthracinus
E. asperulus
E. cyanosporus
E. decipiens
E. granulatus
E. maculatus
E. muricatus
E. mutabilis
E. papillatus
E. persoonii
E. septatus
E. virgatosporus
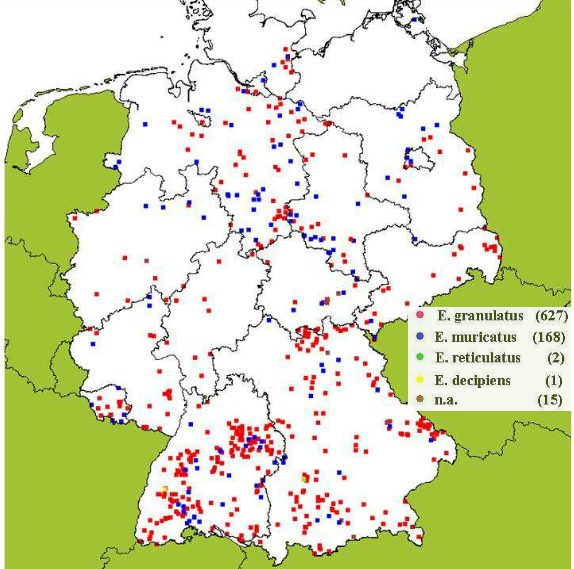
Germany is thought to have 13 species, with predominantly Elaphomyces granulatus (Small-bodied or Warty Deer Truffle), and to a lesser extent Elaphomyces muricatus (Spiny Deer Truffle) found. The distribution map given in Figure 5 is mainly based on locality data provided by the German Society of Mycology for a research project (Fielitz and Richter 2005) and on own locality data. It becomes clear that E. reticulatus and E. decipiens were found only very rarely.
Fig. 5: Distribution map or locality of Elaphomyces spec. in Germany. The legend indicates the species of deer truffles and the number of finds. Data origin: dgfm-ev.de and own localities. Source: Fielitz and Richter 2013
Global distribution
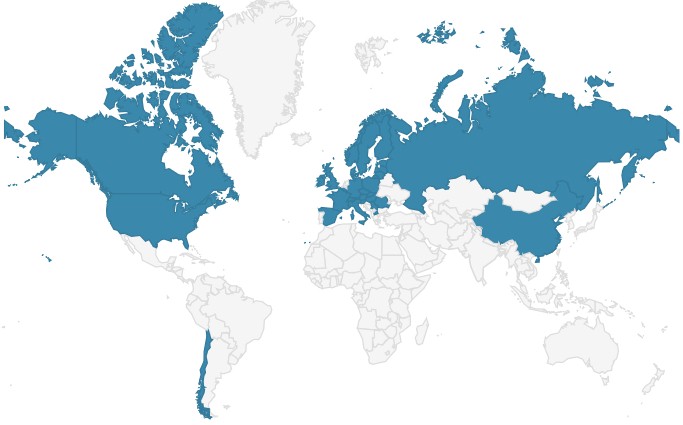
The figure 6 shows the distribution of Elaphomyces granulatus. With the exception of Chile, the species only occurs north of the equator.
Fig. 6: Map of the distribution of deer truffles on earth. Source: iucn.ekoo.se
Australia
Several species of deer truffles also occur in Australia (Figure 5). A total of 299 localities of 14 Elaphomyces species are known. These include E. japonicus, E. leucosporus, E. leveillei, E. morettii, and E. viridiseptum. However, additional species may be present, as indicated by new finds in recent times (Castellano et al. 2012).
Research
Interestingly, little is known about the ecology of Elaphomyces species. Most of the work is from North America, where E. granulatus is one of the dominant species in some forests in terms of biomass: e.g., https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5645184/ “Elaphomyces (‘deer truffles’) is one of the most important ectomycorrhizal fungal genera in temperate and subarctic forest ecosystems, but also one of the least documented in public databases…… and most common European species are still poorly documented.”
We have also addressed the Cs-137 activity of E. granulatus and E. muricatus in two research projects related to radiocesium contamination of wild boar. Currently, we are interested in the following topics:
Inventory & biomass determination
How much biomass of fruiting bodies do mycelia of deer truffles produce? The amount is important for estimating the contribution to radiocesium contamination of feral pigs. No data on fruiting body biomass can be found in the literature.
To determine the inventory of deer truffle fruiting bodies, a defined soil volume is completely searched for truffles. The starting point is in each case a finding place (see figure 5). Our own investigations in the Harz Mountains yielded up to 300 g / kg of fresh weight at high-yield sites. Here we have published information on the conversion to dry matter. We also conducted a deer truffle biomass inventory in the Bavarian Forest National Park on plots of 2m x 2m down to a soil depth of 20 cm (Figure 7).
Fir. 7: Field investigation to determine the biomasses of deer truffles. Germany, Bavarian Forest National Park 2019
Research project inventory of Elaphomyces
In the research project “Erfassung der aktuellen Kontaminationssituation bei Wildschweinen in Deutschland “, the biomass and the radiocesium activity of deer truffles (Elaphomyces spec.) were investigated from 2017 to 2020.
Range and depth distribution of Elaphomyces mycelia
According to Agerer 2001, E. granulatus belongs to the “short-distance exploration type.” The mycelium should only extend over a distance of 1 – 2 cm (Agerer oral). We are trying to verify this information.
Root-associated fungal communities are important components of ecosystem processes that influence plant growth and vigor by affecting the quality, direction, and flow of nutrients and water between plants and fungi.
Ectomycorrhizal fungi frequently associate with forest tree roots, where they enhance nutrient and water uptake, promote seedling establishment, and play an important role in forest nutrient cycling. Predicting the response of ectomycorrhizal fungi such as deer truffles to environmental changes is an important step in maintaining forest productivity in the future. These predictions are currently limited by an incomplete understanding of the relative importance of environmental factors in determining the composition of ectomycorrhizal (ECM) fungi at large spatial scales.
For radiocesium uptake in fruiting bodies of Elaphomyces, their depth distribution is important in the context of mycelial extension. To analyze patterns of vertical mycelial extension, soil sample profiles were analyzed by sequencing the ITS rDNA region. Therefore, at fruiting body sites, we set out to analyze the distribution of mycelia in soil profiles. For this purpose, samples are taken from 2 cm soil layers for DNA analysis and analyzed for species affiliation.
A better understanding of ectomycorrhizal symbiosis is leading to numerous advances in forest management and environmental protection. Morphological identification of ectomycorrhizae often proves misleading. For this reason, a number of molecular methods requiring the isolation of nucleic acids are used to study ectomycorrhizal fungal communities. Ectomycorrhizal root tips are a heterogeneous material with low mass and often preparation for DNA isolation is problematic. Published studies often include a number of unidentified root tips in their results, although several isolation protocols are available to researchers.
Pharmacology
Natural remedies are becoming more and more popular and important among the public and scientific community. In the past, natural remedies have been shown to have interesting biological and pharmacological activity and have been used as chemotherapeutic agents. Truffles are also of increasing interest to pharmaceutical laboratories and research institutions.
Truffles are considered a great delicacy worldwide due to their unique flavor and high nutritional value. To provide information for their potential applications in medicine as well as in functional foods, scientists are studying bioactive components of truffles such as phenols, terpenoids, polysaccharides, arachidonylethanolamide, fatty acids and ergosterols.
The focus is on compounds that have anti-cancer, anti-inflammatory, antioxidant, anti-hyperglycemic, anti-apoptotic, and immunomodulatory effects. Research is also being conducted into those with hepatoprotective and nephroprotective effects.
Cyclooxygenase-2 inhibitory and antioxidant compounds
In deer truffles, cyclooxygenase-2 inhibitory and antioxidant compounds of E. granulatus have been scientifically analyzed so far (Stanikunaite et al. 2009). Cyclooxygenase-2 (COX-2) enzyme plays an important role in inflammatory processes. The authors were able to detect syringaldehyde, a derivative of benzaldehyde, and syringic acid, a hydroxybenzoic acid, among other compounds, from extracts of fruiting bodies. Both compounds substances have shown anti-inflammatory activity in vivo (Fernandez et al., 1998; Gamaniel et al., 2000).
Reactive oxygen species (ROS) and oxidative stress play an important role in the etiology and progression of human degenerative diseases. ROS are involved in inflammation, aging, cancer, heart disease, and diseases other disorders. Antioxidants act as free radical scavengers and are important in protecting against oxidative tissue damage in vital organs.
The authors conclude, deer truffle (E. granulatus) appears to have potential as an agent to combat various diseases due to its antioxidant and anti-inflammatory effects.
Life cycle
The life cycle is largely unknown.
Heavy metals – analysis results
Do fruiting bodies of this hypogeous species accumulate heavy metals? Analysis of a composite sample of fruiting bodies of Elaphomyces granulatus yielded the following values:
Trockenmasse 45,28%
| Lead | Cadmium | Chromium | Nickel | Cooper | Zinc |
| in mg/kg Dry Mass |
|||||
| 1,41 | 1,21 | 0,3 | 114,38 | 1,14 | 224,85 |
und
| Lead | Cadmium | Chromium | Nickel | Copper | Zinc |
| in mg/kg Fresh Mass | |||||
| 0,639 | 0,548 | 0,14 | 51,79 | 0,51 | 101,81 |
Concentrations of lead and cadmium would be of concern to humans at an intake of 30 g per day.
Arsenic
Researchers from the University of Graz and the Academy of Sciences of the Czech Republic in Rozvojová found elevated concentrations of arsenic in Elaphomyces Arte: The research project analyzed 9 collections of fruiting bodies from the Czech Republic using inductively coupled plasma mass spectrometry (ICPMS) and high-performance liquid chromatography coupled with ICPMS. Total arsenic concentrations ranged from 12 to 42 mg/kg dry weight in samples of E. asperulus and from 120 to 660 mg/kg dry weight in E. granulatus and E. muricatus. While higher arsenic concentrations are known in marine organisms, these levels are remarkably high for terrestrial organisms. Deer truffles appear to have significant arsenic accumulation capacity.
The dominant arsenic species in all samples was methylarsonic acid, which accounted for more than 30% of the extractable arsenic. The researchers found high concentrations of trimethylarsine oxide in all samples (0.32-28% of extractable arsenic). This was the first time these arsenic compounds were detected in significant concentrations in terrestrial organisms. Source: Braeuer et al. 2018
Images
The following images show deer truffles in the laboratory prior to sample preparation for gamma spectrometric activity determinations.
Figure 8 shows the size of the fruiting bodies, although these are already larger specimens. In the juvenile stage, the fruiting bodies are only the size of a pin or pea.
Fig. 8: Elaphomyces granulatus with and without root sheaths. With 3.5 cm, the truffle in the center of the picture is already a large fruiting body.
In figure 9, part of the root envelope surrounding the fruiting body has been removed.

Fig. 9: Deer truffle with half-opened root sheath.
How and where to find deer truffles?
The easiest place to find fruiting bodies of the warty deer truffle is at rooting sites of wild boars (Fig. 10). However, wild boars often burrow for other food items, such as rhizomes or protein-rich insect larvae.
Fig. 10: Deer truffles can be found, for example, at burrowing sites of wild boars.
Perseverance is needed in the search. Rubber gloves are helpful, also a screwdriver to loosen the forest floor. Mostly deer truffles are found in the supporting humus.
Elaphomyces species are not listed in the “Ordinance on the Protection of Species of Wild Fauna and Flora” (Bundesartenschutzverordnung – BArtSchV). However, commercial searching is not allowed for deer truffles either. For the search then a nature conservation exemption is necessary, responsible is the lower nature conservation authority.
Truffle dogs
Like the real truffles, deer truffles are also searched for with dogs. For this, however, the dogs must be specially trained. Interested parties can take a course or seminar with their dog for truffle dog training. During the training, the dog owner is first taught the theoretical context of the ecology of the truffle. Then, in the field, the dog is challenged and introduced to truffle hunting. This does not happen overnight. The dog is conditioned to the smell of the deer truffle and later with the finding of a fruit body in direct connection with a reward. The process must be trained again and again until the beginner becomes a real truffle dog.
Purchase and price
Unlike real truffles, there is no market for deer truffles. You can not buy them and thus they have no public price. The reason for this lies in the utility value: there is no use, culinary or otherwise, for the fruiting bodies so far.
Moreover: deer truffles, as described, may not be sought commercially. This eliminates any commercial use. This is because, unlike the valuable true truffle species, cultivation of Elaphomyces has not yet been successful.
Cultivation, cultivation, breeding
Tuber and other real truffles have been cultivated in truffle plantations for years. Truffle cultivation has become a lucrative industry. And truffle nurseries sell roots of host trees inoculated with spores and mycelium of Périgord truffle (Tuber melanosporum) Burgundy truffle (Tuber aestivum var.uncinatum) and Black Perigord truffle (Tuber melanosporum). Inoculated are mainly hazelnut, beech or English oak.
Deer truffles could not be cultivated so far. Rees (1887) tried for a long time to get spores of E. granulatus to germinate, without success. “The same lack of results characterizes all my other attempts to induce spores to germinate or mycelia to transfer to pine roots. In the first respect I have tried at different temperatures, light and dark:dung decocote, fruit decocote, soil extracts, pancreatin and other fermentation solutions, All in vain.”
Not edible or edible but little taste
For humans, the fruit bodies are not palatable. The most likely candidates for a taste test are young fruiting bodies without mature spore development. We taste-tested the still light fruit mass of some young deer truffles: The mass reminds haptically of a thick cream and tastes of nothing. The rind is rubbery, but can be chewed quickly. The taste goes in the direction of earthy forest floor. No wonder!
However, it is not at all known whether deer truffles are possibly poisonous to humans. Fruit bodies with mature spores are largely hollow inside and lined with a blue-black dry, filamentous spore mass and probably not tasty.
History, naturopathy, healing properties and aphrodisiac
In the 21st century, deer truffles have no use in naturopathy, mycotherapy or medicine. This was not always so, because fruiting bodies played in past centuries quite a role as a remedy, in traditional Chinese medicine (TCM) and generally as an aphrodisiac.
The fruiting bodies of Elaphus granulatus and Elaphus muricatus were often found near the rutting grounds of deer. Hence the names “deer truffle”, “deer truffle” and “Fungus cervinus” or “Elaphomyces cervinus”. People therefore assumed a corresponding stimulating effect of the sexual function of deer.
Such discoveries were described several times in past centuries, for example by Hildegard von Bingen in 1180 and by Lonicer in 1577. Hildegard von Bingen, who also dealt with gynecology and childbirth in connection with medicinal plants and mushrooms in her medicinal works, however, also warned against the ingestion of De hirtzswam (deer truffles), saying that they could lead to miscarriages.
Powdered fruit bodies should also help against flatulence.
Means for triggering the rut in farm animals, medicinal plant and drug
The supposed connection between mushroom consumption and sexual function in deer was simply transferred to farm animals. Thus, dried deer truffle preparations were sold as a potency, fertility, or rutting trigger in pigs and cattle, and played a role in veterinary medicine of the time as a medicinal plant and drug.
The way as an aphrodisiac also for humans was then at that time not far. Thus, corresponding deer truffle preparations were offered in pharmacies to increase the desire for love.
Anecdotally, aphrodisiac properties were attributed to the fruit bodies in recent centuries and sold as medicines ( Bauhin 1651, Tulasne & Tulasne 1841 ). Cázares et al. reported the use of decoctions of E. muricatus as a stimulant for “staying young and treating severe wounds” in Mexico, which included its use in shamanic practices. It has been found to have sacred uses in Mexico (GUZMÁN 2008) and has been used as an aphrodisiac in Europe (Hawker 1954).
Site requirements and abundance of Elaphomyces granulatus
E. granulatus (deer balls, hart’s balls, warty deer truffle) is mainly distributed in the northern hemisphere and has been found in Europe, North America, West Asia and Japan (Pegler et al. 1993), there is also evidence from the “East Indies” (Anonymous 1855), today’s Indonesia and Malaysia. It is assumed that E. granulatus can be detected in the entire area of the Fagaceae and Pinaceae (Trappe 1972). The species is one of the most common hypogean mushrooms in Germany (Hensel pers. Comm, Stielow pers. Comm.). The species is also very common in the Netherlands (De Vries 1971), Great Britain (Hawker 1954, Pegler et al. 1993) and Norway (Eckblad 1961) as well as in Poland, Russia and the Baltic States (Bucholtz 1901); in the USA it applies it is the most common underground fungus (Luoma et al. 1991).
In Hungary (Szemere 1965) and Denmark (Lange 1956) the species is not considered very common. E. granulatus is mainly found in Europe among conifers (Bommer & Rosseau 1884, Bucholtz 1901, Jahn 1949, Szemere 1965, Hansen & Knudsen 2000, Hensel pers. Comm., Stielow pers. Comm.), Especially under Pinus sylvestris (Reess & Fisch 1887, Hesse 1894, Fischer 1897, Klugkist 1905, Migula 1913, Jahn 1949, Hawker 1954, Eckblad 1961, De Vries 1971, Benkert 1975, Calonge et al. 1977, Kreisel 1996, Schurig 2002, Schultheis & Tholl 2003) and Picea abies (Fischer 1897, Klugkist 1905, Migula 1913, Eckblad 1961, De Vries 1971, Agerer et al. 2002, Kraigher et al. 2007, Hensel pers. comm., Stielow pers. comm.).
Especially in the spruce forests of the low mountain ranges E. granulatus can be a mass fungus (Stielow pers. comm., Hensel pers. comm., own observations). Rarer are findings among deciduous Quercus species (Hesse 1894, Fischer 1897, Migula 1913, Hawker 1954, Eckblad 1961, De Vries 1971, Benkert 1975, Schultheis & Tholl 2003, Hensel pers. comm.), Fagus sylvatica (Fischer 1897, Migula 1913, Hawker 1954, Lange 1956, Hensel pers. comm.), and Castanea sativa (Migula 1913, Hawker 1954, De Vries 1971).
The variety E. granulatus var. hassiacus was found among Abies alba (Migula 1913). Juniperus communis, Fraxinus excelsior, and Alnus species are reported only from Norway (Eckblad 1961). In the USA, the fungus is also found mainly in coniferous forests. Proven occurrences here (including E. asperulus) are among Abies amabilis, Abies procera, Picea engelmannii, Picea sitchensis, Pinus banksiana, Pinus contorta, Pinus monticola, Pinus ponderosa, Pinus radiata, Pseudotsuga menziesii, Thuja plicata, Tsuga canadensis, Tsuga diversifolia, Tsuga heterophylla, and Tsuga mertensiana (Luoma et al. 1991, Maia et al. 1996, North et al. 1997, North & Greenberg 1998, Gomez et al. 2003, Gomez et al. 2005). Since some of these tree species are cultivated in Germany, occurrences of E. granulatus are also possible here. In Denmark, the species is only recorded under Fagus sylvatica. However, there are records of E. asperulus under conifers (Lange 1956).
E. granulatus tends to be an acidophilous species, occurring mostly on light silicate soils (often sand) (Hesse 1894, Hawker 1954, De Vries 1971, Kreisel 1996, Hensel pers. comm.). However, there are also finds from neutral to slightly basic weathering diabase substrates (Hensel pers. comm.). Hesse (1894) also gives finds on heavy calcareous soils. Eckblad (1961), on the other hand, mentions a greater abundance on calcareous soils than on silicate soils for Norway. The species has a more (north)western distribution in Europe. According to Eckblad (1961), E. granulatus prefers a slightly oceanic climate, which is also supported by the frequent finds in the low mountain spruce forests.
Site requirements and abundance of Elaphomyces muricatus
E. muricatus (incl. E. reticulatus, E. variegatus) occurs in large parts of Germany, but is rarer compared to E. granulatus (Hensel pers. comm., Stielow pers. comm.). However, it is considered the most common Elaphomyces species in Mecklenburg-Vorpommern (Kreisel (1996). Outside Germany, the species is considered common in the Netherlands (De Vries 1971), Great Britain (Hawker 1954, Pegler et al. 1993), and Hungary (Szemere 1965). It is considered less common in Norway and Denmark (Lange 1956, Eckblad 1961). However, Lange (1956) gives E. variegatus as the most common Elaphomyces species in Denmark. The species is listed by all other authors as synonymous with E. muricatus. The species is also rarer than E. granulatus in the USA (Luoma et al. 1991, North et al. 1997, North & Greenberg 1998), although still more common than most other hypogeous fungi. E. muricatus is found primarily under deciduous trees in Europe (Bommer & Rosseau 1884, Bucholtz 1901, Eckblad 1961, Szemere 1965, Hansen & Knudsen 2000).
Most frequent among these are finds under Fagus sylvatica (Hesse 1894, Fischer 1897, Migula 1913, Hawker 1954, E. variegatus in Lange 1956, De Vries 1971, Pegler et al. 1993, Schultheis & Tholl 2003, Kraigher et al. 2007, Hensel pers. comm., Stielow pers. comm.), but also deciduous Quercus spp. (Hesse 1894, Fischer 1897, Migula 1913, De Vries 1971, Benkert 1975, Hensel pers. comm., Stielow pers. comm.) and Pinus sylvestris (Reess & Fisch 1887, Hesse 1894, Migula 1913, Eckblad 1961, Benkert 1975, Schultheis & Tholl 2003). Furthermore, Betula spp. (Hesse 1894, Eckblad 1961, Pegler et al. 1993, Hansen & Knudsen 2000, Schurig 2002), Castanea sativa (Hesse 1894, Fischer 1897, Migula 1913) and Picea abies (Lange 1956, Eckblad 1961) are given. Sporadically, the species has been recorded among Abies alba (De Vries 1971), Corylus avellanus (Hensel pers. comm.), Juniperus communis, and Alnus species (Eckblad 1961).
In the United States, E. muricatus (including E. variegatus and E. reticulatus) has been found in forests with Abies amabilis, Picea sitchensis, Pinus ponderosa, Pseudotsuga menziesii, Thuja plicata, Tsuga heterophylla, and Tsuga mertensiana (Luoma et al. 1991, Maia et al. 1996, North et al. 1997, North & Greenberg 1998, Gomez et al. 2003, Gomez et al. 2005). E. muricatus seems to prefer two habitats: in Mecklenburg-Vorpommern, Brandenburg, and in Denmark, finds on acid soil are common (Lange 1956, Benkert 1975, Kreisel 1996), in northeastern Germany in pine-oak forests (Pino-Quercetum), and in Denmark in acid beech forests (as E. variegatus) and spruce forests. Sandy soil is also given for the Netherlands (De Vries 1971).
In contrast, the species is common in central Germany [Hesse, southern Lower Saxony, Saxony-Anhalt, Saxony, Thuringia] (Hensel pers. comm., Stielow pers. comm.) and Great Britain (Hawker 1954, Pegler et al. 1993) rather on alkaline soils, and especially in calcareous beech forests with rendzina soils (Stielow pers. comm.). The species also occasionally occurs in Germany together with E. granulatus (Hesse 1894). Concerning the occurrence in coniferous forests, Pegler et al. (1993) state that these are always plantations on old deciduous forest sites. However, this is not true at least for the findings in northeastern Germany, the pine and pine-oak forests there are considered climax forests. The species has a more southern to southeastern distribution in Europe compared to E. granulatus (Eckblad 1961, Szemere 1965) and possibly prefers a climate with a slightly continental character.
News
12/20/20 On the Sunday walk in mid-December 2020, the forest was full of burrows. The first deer truffle was found after a short time, more were to follow…..
29.03.2020 In the 2020 budget of the Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, 330,000 euros have been allocated for compensation payments to meet compensation claims under Section 38 (2) of the Atomic Energy Act as a result of the Chernobyl accident.
The compensation payments are made to hunters for game contaminated by radioactivity from the Chernobyl fallout. Source: BMU.
“Currently, due to the food habits of animals, wild boars are still mainly affected (ingestion of deer truffles contaminated with cesium). The development of the amount of compensation claims is therefore primarily derived from the wild boar population and the respective hunting distance, which have increased significantly in recent years as a result of mild winters and an increasing food supply (corn monocultures). Fluctuations due to weather conditions, e.g., in colder years, are possible.”
Mild winter 2019/2020 favors deer truffle growth
02/19/2020 update.
01/30/2020 Mild winter weather favored deer truffle growth in the winter of 2019/2020. Temperatures were below long-term average temperatures in December 2019, January, and February 2020 (reference). In addition, according to the European Centre for Medium-Range Weather Forecasts (ECMWF), deposited precipitation is also higher than usual in many regions of Germany.
On our field trips in January we found significantly more fruiting bodies of deer truffles than the long-term average. These are specimens that have grown in the last few months. Meanwhile we found fruiting bodies of all ages. Thereby also large specimens of 10 g with blue-purple, mature spores.
Literature
Agerer R. (2001): Erkundungstypen von Ektomykorrhizen. Ein Vorschlag zur Klassifizierung von Ektomykorrhiza-Myzelsystemen nach ihren Differenzierungsmustern und ihrer mutmaßlichen ökologischen Bedeutung. Mycorrhiza 11(2): 107 – 114. Auszug online verfügbar auf https://link.springer.com/article/10.1007/s005720100108.
Braeuer, S., Borovička, J., Goessler, W. 2018:A unique arsenic speciation profile in Elaphomyces spp.-trimethylarsine oxide and methylarsonous acid as significant arsenic compounds. Analytische und Bioanalytische Chemie. 410 (9): 2283–2290. Zusammenfassung
Castellano M. A., Henkel T. W., Miller S. L. , Smith M., Aime M., 2012. Neue Elaphomyces-Arten (Elaphomycetaceae, Eurotiales, Ascomycota) aus Guyana. Mycologia. 104 (5): 1244?9. doi:10.3852/12-061. Link
Cázares, E.; Trappe, J.M. 1994. Sporenausbreitung von Ektomykorrhizapilzen auf einem Gletscher durch Säugetier-Mykophagie. Mycologia. 86: 507-510.
Fernandez, MA.,, Saenz, MT., Garcia, MD. 1998: Entzündungshemmende Aktivität von aus Scrophularia frutescens isolierten Phenolsäuren bei Ratten und Mäusen. J Pharm Pharmacol 50: 1183-1186.
Gamaniel, K., Samuel, BB., Kapu, DS et al.: 2000: Anti-Sickling, analgetische und entzündungshemmende Eigenschaften von 3, 5-Dimethoxy-4-hydroxybenzoesäure und 2, 3, 4-Trihydroxyacetophenon. Phytomedicine .
7: 105–110.
Fielitz, U., Richter K., 2013: Bundesweiter Überblick über die Radiocäsiumkontamination von Wildschweinen : Abschlussbericht zum Forschungsvorhaben 3607S04561 / online verfügbar: https://doris.bfs.de/jspui/bitstream/urn:nbn:de:0221-2013102411098/3/BfS_2013_3607S04561.pdf
Gold, C. 2019: Die Gattung Elaphomyces – Eine Bestandsaufnahme der Vorkommen in Süddeutschland. Mycol. Bav. 19:111-14
Hildegard von Bingen: Pflanzliche Heilmittel Mit gynäkologisch-geburtshilflicher Indikation. Zur Medizingesch Abh. 1994;259:1-245
Hildegard von Bingen: Heilkunde
Lonicer A. 1577: Kreuterbuch
Paz, A.; Bellanger, J.-M.; Lavoise, C.; Molia, A.; Ławrynowicz, M.; Larsson, E.; Ibarguren, I.O.; Jeppson, M.; Læssøe, T. (2017-06-30). “The genus Elaphomyces (Ascomycota, Eurotiales): a ribosomal DNA-based phylogeny and revised systematics of European ‘deer truffles'”. Persoonia – Molekulare Phylogenie und Evolution der Pilze. 38 (1): 197-239. doi:10.3767/003158517×697309
Rees M. 1887: Untersuchungen über Bau u. Lebensgeschichte der Hirschtrüffel, Elaphomyces.
Reynolds H., T., 2011: Systematik, Phylogeographie und Ökologie der Elaphomycetaceae. Duke University. Dissertation.
Schickmann S, Urban A, Kräutler K, Nopp-Mayr U, Hackländer K. 2012 -Die Wechselbeziehung von mykophagen Kleinsäugern und Ektomykorrhizapilzen in urzeitlichen, gestörten und bewirtschafteten mitteleuropäischen Bergwäldern. Oecologia 170, 395-409.
Stanikunaite, R., Khan, SI., Trappe, JM., Ross, SA. 2009: Cyclooxygenase-2 hemmende und antioxidative Verbindungen aus der Trüffel Elaphomyces granulatus. Phytotherapy Research Apr;23 (4):575-8. Artikel hier einsehbar.
https://www.fs.fed.us/pnw/pubs/journals/pnw_2008_stanikunaite001.pdf
Agerer, R. (2001): Exploration types of ectomycorrhizae. Mycorrhiza 11: 107-114.
Agerer, R., Grote, R., Raidl, S. (2002): The new method ‘micromapping’, a means to study species specific associations and exclusions of ectomycorrhizae. Mycol. Progress 1 (2): 155-166.
Anonymous (1855): Donations to the Museum of the Linnean Society. Trans. Linn. Soc. London 21: 347-353.
Benkert, D. (1975): Elaphomyces und Cordyceps in Brandenburg. Gleditschia 3: 189-194.
Blaschke, H., Bäumler, W. (1989): Mycophagy and Spore Dispersal by Small Mammals in Bavarian Forests. Forest Ecology and Management, 26: 237-245.
Bommer, E., Rousseau, M. (1884): Florule Mycologique des Environs de Bruxelles. Bulletin de la société royale de botanique de Belgique 23:15-365.
Bucholtz, F. (1901): Hypogaeen aus Russland. Hedwigia 40: 304-322.
Calonge, F.D., de la Torre, M., Lawrynowicz, M. (1977): Contribición del estudio de los hongos hipogeos de España. Anal. Inst. Bot. Cavanilles 34 (1) 15-31.
Castellano, M.A., Bougher, N.L. (1994): Consideration of the taxonomy and biodiversity of Australian ectomycorrhizal fungi. Plant and Soil 159: 37-46.
De Vries, G.A. (1971): De fungi van nederland. III. Hypogaea. Truffels & schijntruffels. Wetenschappelijke meddelingen koninklijke nederlandse natuurhistorische vereniging 88: 1-63.
Eckblad, F.-E. (1961): Studies in the Hypogaean Fungi of Norway II. Revision of the Genus Elaphomyces. Nytt. Mag. Bot. 9: 199-210.
Fielitz, U. (2005): Untersuchungen zum Verhalten von Radiocäsium in Wildschweinen und anderen Biomedien des Waldes. Bundesministeriums für Umwelt, Naturschutz und Reaktorsicherheit. Schriftenreihe Reaktorsicherheit und Strahlenschutz, BMU, 675.
Fischer, E. (1897): Plectascinae. In: Engler, Prantl (Hrsg.): Die natürlichen Pflanzenfamilien. Bd. 1.1. S.290-320.
Gomez, D.M., Anthony, R.G., Trappe, J.M. (2003): The Influence of Thinning on Production of Hypogeous Fungus Sporocarps in Douglas-fir Forests in the Northern Oregon Coast Range. Northwest Science Abstracts 77 #4: 2.
Gomez, D.M., Anthony, R.G., Hayes, J.P. (2005): Influence of thinning of douglas-fir Forests on Population Parameters and diet of northern flying squirrels. Journal of Wildlife Management 69(4):1679-1682.
Grönwall, O., Pehrson, Å. (1984): Nutrient content in fungi as a primary food of the red squirrel Sciurus vulgaris L. Oecologia (Berlin) 64: 230-231.
Hansen, L., Knudsen, H. (2000): Nordic Macromycetes Vol. 1. Ascomycetes. Kopenhagen.
Hawker L.E. (1954): British hypogeous fungi. Phil. Trans. Roy. Soc. London 237: 429-546.
Helfer, W. (1991): Pilze auf Pilzfruchtkörpern. Untersuchungen zur Ökologie, Systematik und Chemie. Libri Botanici 1. Eching.
Hesse, R. (1894): Die Hypogaeen Deutschlands. Bd. II. Die Tuberaceen und Elaphomyceten. Halle a. S.
Hesse, R. (1989): Zur Entwicklungsgeschichte der Tuberaceen und Elaphomyceten. Bot. Zentralblatt 10: 553-557.
Jahn (1949):Pilze rundum. Hamburg.
Klugkist, C.E. (1905): Discomyceten, Elaphomyceten und Gasteromyceten aus Nordwestdeutschland. Abhandlungen des Naturwissenschaftlichen Vereins zu Bremen 18: 376-383.
Kotter, M.M., Farentinos, R.C. (1984): Formation of Ponderosa pine ectomycorhiza after inoculation with feces of tassel-eared squirrels. Mycologia 76 (4): 758-760.
Kraigher, H., Petkovšek, S.A.S., Grebenc, T., Simončič, P. (2007): Types of Ectomycorrhiza as Pollution Stress Indicators: Case Studies in Slovenia. Environ. Monit. Assess. 128:31-45.
Kreisel, H. (1996): Hirschtrüffeln – Stiefkinder der Mykologen? Botanischer Rundbrief für Mecklenburg-Vorpommern 29: 163-166.
Lange, M. (1956): Danish Hypogeous Macromycetes. Dansk Bot. Ark. 16 (1): 5-84.
LoBuglio, K.F., Berbee, M.L., Taylor, J.W. (1996): Phylogenetic Origins of the Asexual Mycorrhizal Symbiont
Cenococcum geophilum Fr. and Other Mycorrhizal Fungi among the Ascomycetes. Mol. Phyl. Evol. 6 (2): 287-294.
Luoma, D.L., Frenckel, R.E., Trappe, J.M. (1991): Fruiting of hypogeous fungi in Oregon Douglas-fir forests: seasonal and habitat variation. Mycologia 83 (3): 335-353.
Luoma, D.L., Trappe, J.M., Claridge, A.W., Jacobs, K.M., Cázares, E. (2003): Relationships among fungi and small mammals in forested ecosystems. In: Zabel, C.J, Anthony, R.K. (Hrsg.): Mammal Community Dynamics. Management and Conservation in the Coniferous Forests of Western North America. Cambridge, New York, Singapur: 343-373.
McKay, M.D., Conover, W.J. and Beckman, R.J. A. (1979): Comparison of Three Methods for Selecting Values of Input Variables in the Analysis of Output From a Computer Code. Technometrics 21, 239–245.
Maia, L.C., Yano, A.M., Kimbrough, J.W. (1996): Species of Ascomycota forming ectomycorrhizae. Mycotaxon 57: 371-390.
Maser, C., Trappe, J.M., Nussbaum, R.A. (1978): Fungal-small mammal interrelationships with emphasis ob Oregon coniferous forests. Ecology 59 (4): 799-809.
Meyer, M.D., North, M.P. (2005): Truffle abundance in riparian and upland mixed conifer forest of California’s southern Sierra Nevada. Can. J. Bot. 83:1015-1020.
Migula, E.F.A.W. (1912): Kryptogamen-Flora. Bd. III. Pilze. 3.Tl. 1.Abt. In: Direktor Prof. Dr. Thomé’s Flora von Deutschland, Österreich und der Schweiz. Bd.X.Abt.1. Gera.
North, M., Trappe, J.M., Franklin, J. (1997): Standing crop and animal consumption of fungal sporocarps in Pacific Northwest forests. Ecology 78 (5): 1543-1554.
North, M. Greenberg, J. (): Stand conditions associated with truffle abundance in western hemlock/Douglas-fir forests. Forest Ecology and Management 112: 55-66.
Pegler, D.N., Spooner, B.M., Young, T.W.K. (1993): British truffles. A revision of British hypogeous fungi. Kew.
Rancken, H. (1910): Korrektur zu Med. Soc. pro F. et Fl. Fenn. 34 p. 112. Meddelanden af Societas pro Fauna et Flora Fennica 43: 4.
Reddell, P., Spain, A.V. (1991): Earthworms as vectors of viable propagules of ectomycorrhizal fungi. Soil Biol. Biochem. 23 (8): 767-774.
Reess, H., Fisch, C. (1887): Untersuchungen über Bau und Lebensgeschichte der Hirschtrüffel, Elaphomyces. Abhandlungen aus dem Gesammtgebiete der Botanik 7: 1-24.
Schultheis, B., Tholl, M.-T. (2003): Journées luxembourgoises de mycologie vernale 2001. Bull. Soc. Nat. Luxemb. 104: 21-39.
Schurig, B. (2002):„Trollhand“ und Anis-Sägeblättling bei Ludwigslust gefunden. Mitteilungen der NGM 2 (2): 110.
Sung, G.-H., Hywel-Jones, N.L., Sung, J.-M., Luangsa, J.J., Shrestha, B, Spatafora, J.W. (2007): Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol 57: 5-59.
Szemere, L. (1965): Die unterirdischen Pilze des Karpatenbeckens. Budapest.
Trappe, J.M. (1972) Mycorrhiza-forming Ascomycetes. In: Mycorrhizae. Proc. 1st NACOM April 1969, Illinois. Ed. E. Hacskaylo. pp 19-37. USDA For. Serv. Misc. Publ. Zit in Castellano & Bougher 1994.
http://www.dur.ac.uk/resources /its/info/guides/94Avenue.pdf (Juli 2008)