Cesium-137 is a gamma emitter with a half-life of 30.08 years. Cesium is a soft, gold-colored metal with a melting point of 28.5 °C. It has atomic number 55 and a relative atomic mass of 132.9.There are 35 known isotopes of the element cesium: Cesium-114 to Cesium-148 (Seelmann-Eggebert et al. 1981). When we talk about radiocesium, we mean cesium-137.
Stable cesium 133 (Cs-133) is the only naturally occurring isotope. It is found mainly in the mineral pollucite, containing up to 30% Cs20. All other cesium isotopes are formed artificially, in atomic bomb explosions and in nuclear reactors, including the long-lived cesium 135.
Overview
Here are some important facts at a glance
| Caesium 137 | |
| Radiation type | Gamma rays, beta rays |
| physical half-life | 30.19 years |
| biological half-life human | approx. 70 – 110 days |
| effective half-life human | approx. 69 – 109 days |
| Accumulation in | Muscle tissue |
| Input into the environment significant | Global nuclear weapons fallout |
| Chernobyl fallout | |
| Fukushima accident | |
| Limit value* for milk and milk products | 370 Bq / kg (EU, Germany) |
| Limit value for other foodstuffs | 600 Bq / kg (EU, Germany) |
| limit value exceeded, regionally partly | Mushrooms, wild boar meat |
*=valid for total cesium (Cs-134 + Cs-137)
Radiocesium: Cesium 137 is relevant for radiation biology
From a radiobiological point of view, Cs-137 is the most important Cs isotope. With a fission yield of 6.2%, it is produced in relatively large quantities in atomic bomb explosions (KATCOFF 1958) and, with a physical half-life of about 30 years, remains in the environment over the long term. The decay scheme of the nuclide is shown in the figure 1
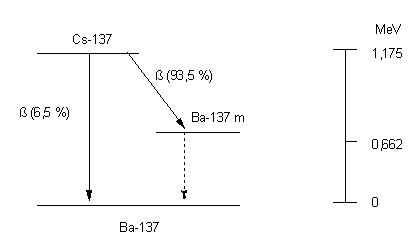
Half-life 11018.30 days = 30.187 years
Cesium-137 has a long half-life of 30.19 years based on a human life span, while the activity of iodine-131, for example, is halved within 8 days due to radioactive decay.
From a radiobiological point of view, besides Cs-137, cesium-134 is also of less importance. Cs-134 decays with a physical half-life of 2.1 years with the emission of beta and gamma rays. It is mainly formed by neutron capture on the stable Cs-133 in nuclear reactors, while it is only formed in traces in atomic bomb explosions.
Just like potassium, cesium belongs to the alkali metals and is the most ignoble and therefore most reactive element of this group. It reacts chemically and metabolically-physiologically similar to potassium (Davis 1963), which is essential for many organisms and is enriched intracellularly. However, cesium cannot replace potassium in its metabolic functions and is therefore usually not absorbed by organisms in the same proportion as potassium (Kornberg 1961). The reason for this could be the different ionic radii: they are + 1.33 Å for K and + 1.65 Å for Cs. A biological significance of Cs for animals or plants has not yet been proven.
Storage in the human body
The nuclide is mainly ingested with food and is distributed in the organism mainly in muscle tissue, very similar to potassium-40. In contrast to strontium 90, the incorporation into bone tissue is low. Due to the metabolic activity, cesium-137 is excreted in the human body with a biological half-life of approx. 110 days.
Gamma spectrum
The figure shows the excerpt relevant for Cs-137 from the gamma spectrum of a sample measurement of deer truffle on a pure germanium detector at the Laboratory for Radioisotopes (LARI) at the University of Göttingen.
The measured pulses are plotted on the ordinate and the energy is plotted on the abscissa.
The main maximum of the gamm energy distribution is 662 keV (Full Energy Peak). The percentage yield per disintegration is 85%.
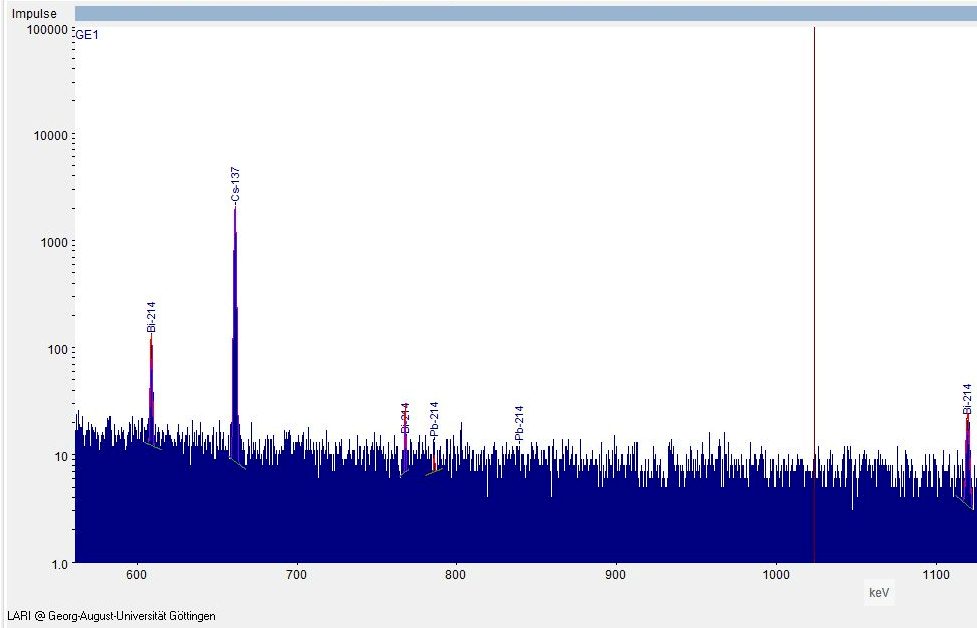
Fig. 2: Gamma spectrum with cesium-137 peak from a measurement on deer truffle. Source: LARI
Limit values 370 Bq / kg or 600 Bq / kg
For cesium 137 there is a limit of 600 Bq per kilogram for food (except milk) in the trade, but this does not apply to personal use. For example, hunters are allowed to consume meat from a hunted wild boar for their own consumption even with activities above 600 Bq / kg.
The limit values are specified in Council Regulation EEC 737/90 of March 22, 1990 on the import conditions for agricultural products originating in third countries after the accident at the Chernobyl nuclear power plant.
The limit values were also retained in the regulation of 2008 (Regulation (EC) No. 733/2008) and are valid until March 31, 2020 (extract):
“Article 2
1. Without prejudice to the other provisions in force, the products referred to in Article 1 may only be released into free circulation on condition that the maximum levels laid down in paragraph 2 of this Article are observed.
(2) The maximum cumulative radioactivity of cesium-134 and cesium-137 must not exceed the following values (9):
a) 370 Bq / kg for milk and milk products, which are listed in Annex II, as well as for foodstuffs for the nutrition especially of infants during the first four to six months of life, which in themselves meet the nutritional needs of this group of people and in packs for retail clearly marked and labeled as preparations for infants;
b) 600 Bq / kg for all other affected products. ”
The European Union also mentions the special importance of biomedia in forest ecosystems in Council Regulation (EC) No. 1048/2009 of 23 October 2009:
“(3) There is scientific evidence that the duration of cesium-137 contamination after the Chernobyl accident in the case of a number of products originating from species living or growing in forests and wooded areas essentially depends on the half-life this radionuclide (30 years) is dependent.
(4) Regulation (EC) No 733/2008 should therefore be amended accordingly – … .. ”
Change in 2011: After the accident at the Fukushima nuclear power plant, the EU changed the limit values through the (EU) No. 351/2011 regulation and raised the maximum values:
COMMISSION IMPLEMENTING REGULATION (EU) No. 351/2011
of April 11, 2011 amending Regulation (EU) No. 297/2011 on the adoption of special provisions for the import of food and feed originating from or coming from Japan after the accident at the Fukushima nuclear power plant
Switzerland
Until 2016, the limit value for cesium 137 in food in Switzerland was 1200 Bq / kg. Then in 2016 the existing limit values should be completely abolished. However, this was not implemented after protests, but adjusted to the EU level:
With the ordinance of 16 December 2016 “FSVO on the importation and placing on the market of foodstuffs contaminated with cesium due to the accident at the Chernobyl nuclear power plant (Chernobyl Regulation) “this maximum value is set:
370 Bq / kg for: Milk and milk products (sour milk, acidified or fermented milk, cream, as well as cream / sour cream, buttermilk, yoghurt, kefir and whey)
370 Bq / kg for food intended for infants up to six months old
600 Bq / kg for all other foods (including game).
Further details are available from the Swiss Federal Council.
Research
More than 30 years after the Chernobyl accident, increased radiocesium contamination is largely limited to some biomedia in forest ecosystems, such as mushrooms and wild boars. Accordingly, research today is mainly carried out in this area. Questions are still the dynamics of radiocesium in forest soils, fungi and wild animals as well as corresponding modeling. Umweltanalysen.com has been conducting its own research and contract research in these areas since 1994, mainly for the BfS.
A new study provided a map of the origins of plutonium and cesium-137 (Meusburger et al. 2020): Global nuclear weapons tests and the Chernobyl accident released large amounts of radionuclides into the environment. To date, however, the spatial patterns of these fallout sources are hardly restricted. Fallout radionuclides (Cs 137, Pu 239, Pu 240) were measured in soil samples (n = 160) that were collected in flat, undisturbed grasslands in Western Europe as part of a harmonized European soil survey. The authors show that both fallout sources left a specific radionuclide footprint in European soils.
They used plutonium to quantify the contributions of global versus Chernobyl precipitation to Cs-137 in European soils. Spatial predictive models enabled an initial assessment of the global fallout pattern compared to Chernobyl across national borders. Understanding the size of these fallout sources is important not only to establish a baseline for future radionuclide fallouts, but also to define a baseline for geomorphological reconstructions of soil redistribution due to soil erosion processes.
Test to determine the age of wines
Philippe Hubert from the Nuclear Research Center Center d’Etudes Nucléaires (CENBG) determined the age of bottled wine by testing it with Cs 137 back in 2009 (Hubert et al. 2009). Using the radioactivity measurements of wine, the scientist and colleagues showed that in addition to the well-known isotope potassium 40, wine also contains traces of cesium 137 with an activity that depends on the vintage. However, the activity is low and is less than 1 Bq per liter. This technology has led to the fact that the wine bottles of the vintages between 1952 and 1980 could be dated and the year indicated on the label or on the cork could be checked. Since the measurements do not require opening the bottle, the technique has also proven to be very useful in detecting counterfeit wines of the XIX century. Century and the first half of the XX: because wine before 1952 cannot contain cesium 137, not even in traces. This makes it easy to identify counterfeits of very old vintages.
Cs-137 in the environment
Since 1945, artificial, radioactive fission products have been released into the atmosphere through atomic bomb tests and spread worldwide. Each test produces around 200 fission products, many of which, due to their short physical half-life, can no longer be detected after a short time. Of the remaining fission products, the isotopes strontium 90 and cesium 137 are of particular importance:
they have long half-lives (Sr 90 = 28 years, Cs 137 = 30 years)
they behave physiologically in a similar way to the important bio-elements calcium and potassium
in every nuclear test they arise in larger quantities (approx. 3-7% fission yield)
Global fallout
In the aboveground atomic bomb tests, the radioactive fission products reached the atmosphere and were deposited worldwide through mass transfer reactions between the troposphere and the stratosphere. This deposition mainly took place with the precipitation, but partly also via dry deposition.
As a result of these above-ground atomic bomb tests in the 1950s and 1960s, the radioactive isotope Cs-137 is still present in environmental media around the world. As the number of nuclear weapons tests increased, the global exposure to Cs-137 increased continuously.
In response to international pressure, the USA, the former USSR, and Great Britain signed a limited nuclear test ban treaty in 1963, which now only allows underground atomic bomb tests. In the following three decades, the global environmental contamination with Cs-137 then slowly decreased.
Global warming and dynamics of cesium 137
Long-term studies on the dynamics of 137Cs in forest ecosystems could meanwhile also have an impact on global warming. The long-term retention of the nuclide in the upper soil layers is also largely due to biotic transport processes. Decline in species of destructors, changes in the formation rates of soil organic matter due to a lack of water and temperature stress could change the depth distribution of 137Cs in the soil as well as the uptake rates of the nuclide by the deer truffle mycelium. With the exception of Winkelbauer (2012), no systematic studies have been carried out on the depth distribution and migration of 137Cs in forest soils in Germany over the past 15 years.
Cesium 133 (Cs 133) a strategically important metal in the high tech age
The 5G revolution, American military defense, and even time itself depend on that one critical metal that China is monopolizing and that the US is desperate to get more of: the metal is cesium. But global dominance, which depends on technological superiority, is not possible without it.
In May 2018, cesium was placed on the list of critical minerals by the United States Department of the Interior. In fact, there are a total of 16 metals that are absolutely critical to high-tech industries, military applications, and telecommunications – and China controls the supply of every single metal because it controls 96% of production.
That includes cesium, for which China has a monopoly on inventory, the mines are no longer really producing, and the United States is no longer, so North America’s only hope is Canada. Cesium is so secret and opaque that it is almost impossible to track its actual market price. It’s strategic in and of itself, but its rarity makes it even more critical.
Global supremacy cannot be achieved without these metals
The supreme technological war of global supremacy cannot be won without these metals, so whoever controls them is in pole position.
Cesium is described by the German Institute for Strategic Metals (ISE) as “the most electropositive of all stable elements in the periodic table” and the heaviest of the stable metals. Cesium is “extremely pyrophoric, ignites spontaneously on contact with air and explodes violently in water or ice at any temperature above -116 ° C”.
Applications of the strategic metal in the healthcare industry are expected to see a surge as laboratories are already using cesium compounds in medical imaging, cancer therapy, positron emission tomography (PET) and more.
Technavio’s latest market analysis predicts that the Cesium market will grow by 1.66 thousand MT between 2020 and 2014, powered by everything from catalyst promoters, glass amplifiers, photoelectric cell components, crystals in scintillation counters, and getters in vacuum tubes.
A great need for cesium also comes from the oil and gas industry, which uses cesium formate brines in drilling fluids to prevent outbreaks in high temperature and overpressure wells.
Key metal
In terms of world supremacy, the “cesium standard” is key. This is the standard by which accurate, commercially available atomic clocks measure time, and it is critical to the data transmission infrastructure of cellular networks, GPS and the Internet.
This means that it is also used in defense products, including infrared detectors, optics, night vision goggles and much more. Investments were even made in a cesium laser for use in missile defense and other technological applications.
If China refuses to export cesium, it will seriously disrupt US industry and hinder the development of critical military equipment. Because of this, the United States and Canada finally agreed in December 2019 on a strategy to reduce the demand for rare earth metals that are mined or controlled by China. Source: https://oilprice.com
Situation in the USA
Domestic production and use: No cesium was mined domestically in 2019. This made the United States 100% dependent on imports of cesium minerals. Pollucite, which is found mainly in connection with lithium-rich, lepidolite-containing or petalite-containing zoned granite pegmatites, is the most important cesium ore mineral. Cesium minerals are used as a starting material for producing a wide variety of cesium compounds and cesium metal. The element’s primary application, on a gross weight basis, is in cesium formate brines, which are used for high pressure and high temperature wells for oil and gas production, and exploration.
Use
Cesium metal is used in the manufacture of cesium compounds and possibly in photoelectric cells. Cesium bromide is used in infrared detectors, optics, photoelectric cells, scintillation counters, and spectrophotometers. Cesium carbonate is used in the alkylation of organic compounds and in energy conversion devices such as fuel cells, magnetohydrodynamic generators, and polymer solar cells. Cesium chloride is used in analytical chemistry as a reagent, in high-temperature solders, as an intermediate in the production of cesium metal, in isopycnic centrifugation, as a radioisotope in nuclear medicine, as an insect repellent in agriculture and in special glasses. Cesium hydroxide is used as an electrolyte in alkaline storage batteries.
Cesium iodide is used in fluoroscopy devices – Fourier transform infrared spectrometers – as the input phosphor of X-ray image intensifier tubes and in scintillators. Cesium nitrate is used as a colorant and oxidant in the pyrotechnic industry, in petroleum cracking, in scintillation counters and in X-ray fluorescent materials. Cesium sulfates are water soluble and are believed to be used primarily in water treatment, fuel cells, and to improve the optical quality of scientific instruments.
Resonance frequency norm in atomic clocks
Cesium isotopes are used as the atomic resonance frequency standard in atomic clocks, which plays a decisive role in aircraft guidance systems, worldwide positioning satellites as well as Internet and mobile phone transmissions. Cesium clocks monitor the cycles of microwave radiation emitted by the electrons in the cesium and use these cycles as a time reference. Due to the high accuracy of the cesium atomic clock, the international definition of 1 second is based on the cesium atom. The U.S.A. civil time and frequency standard is based on a cesium fountain clock from the National Institute of Standards and Technology in Boulder, CO. The U.S. military’s frequency standard, the United States Naval Observatory’s time scale, is based on 48 weighted atomic clocks, including 25 cesium fountain clocks.
Cesium price
United States
Usage, import, and export data for cesium in the United States has not been available since the late 1980s. Since the metal is not traded in commercial quantities, a market price is not available. It is believed that several thousand kilograms of cesium chemicals are used in the United States each year. The United States imports all of its cesium-133 needs.
In 2019, a company offered 1 gram vials of 99.8% (metal-based) cesium for $ 63.00, up from $ 61.80 in 2018, and 99.98% metal-based for $ 81.10, up up 3% from $ 78.70 in 2018.
In 2019, prices for 50 grams of 99.9% (metal basis) cesium acetate, cesium bromide, cesium carbonate, cesium chloride and cesium iodide will be $ 118.20, $ 71.90, $ 101.80, $ 103.60 and 117, respectively. $ 00, an increase of 3% over 2018 prices. The price of a standard cesium plasma solution (10,000 micrograms per milliliter) was $ 81.90 for 50 milliliters and $ 125.00 for 100 milliliters, and the price for 25 grams of cesium formate, 98% basis, was $ 39.90. Source: usgs.gov
Germany
In Germany a “Cesium Metal Element 55 Sample 15 MG Ampoule 99.99% IN Periods Element Tile” is sold on eBay
Sealed in a glass ampoule and periodic element tile offered for 17.47 euros (plus shipping costs) (source: https://peguys.com/collections/periodic-element-samples/caesium). That corresponds to a gram price of 1,165 euros.
Another provider offers “Cesium 50g ampoule, 99.99% purity” for 850 euros including VAT plus shipping costs. (Source: http://www.shop-027.de/Chemicalshop-p3h7s9-Caesium-Cs-Cesium-14.htm).
Via https://german.alibaba.com/product-detail/cesium-133-50012137120.html a minimum purchase quantity of 5 kg Cs 133 is offered: “We sell very high quality Cesium 133 with a high degree of purity. We can deliver as much as poseible, the product from Russia but the port is Bishkek Kyrgzstan. We have the best price for the quality, very competitive and can always visit our company for further confirmation. Call or email us for the best price ever, we are happy to work with you.
Can we get the best price on Cesium 133. “According to the data sheet, the provider comes from” Moscow, Russian Federation ”
News
06/28/2020 The IAEA reports slightly increased cesium-137 activity for several countries in Scandinavia and the Baltic States due to measurements from official measuring points (data source: IAEA information):
The International Atomic Energy Agency is aware of information from the interim technical secretariat of the Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO) that its International Monitoring System (IMS) increased levels of three radioisotopes Ru-103, Cs-134 and Cs-137 in the Air at an IMS monitoring station in Sweden.
In such cases, in accordance with normal practice, the IAEA has asked its colleagues for information on whether these radioisotopes have been detected in their countries and whether an event may have been linked to this atmospheric release.
Already in April 2020 in Northern Norway at a measuring station of the Norwegian Agency for Nuclear Safety (DSA), increased Cs-137 activity on aerosol filters was noticed, albeit in a very low concentration:
“The DSA airborne radioactivity stations in Svanhovd and Viksjøfjell in Finnmark measured microscopic amounts of radioactive cesium for the week of April 12-19. The microscopic amounts pose no danger to humans or the environment.
No radioactive cesium was recorded at other stations in the country.
The DSA’s forecast of airborne radioactivity for the period April 6-16 shows that particles from the Chernobyl area may have reached Finnmark (see figure below). The same is shown by scatter forecasts from the Meteorological Institute. This confirms our theory that there is radioactive cesium from the Chernobyl forest fires that we see at the measuring stations.
DSA will continue monitoring and will publish the measurement results continuously when more stations measure radioactive particles in the future. ”
The authority suspects that radioactive cesium from the forest fires in Chernobyl may have reached Norway.
Spread of cesium 137 from the reactor accident from Fukushima to Alaska:
Since the destruction of the Fukushima Nuclear Power Plant in 2011, many Alaskans have raised concerns about radiation in seawater and marine life. Measurable amounts of radioactive substances have long been present in oceans and seas, including the Bering Sea. These come from a combination of naturally occurring and man-made sources (e.g. nuclear weapons tests and accidental releases from nuclear reactors). Cesium-137 and cesium-134, are byproducts of nuclear fission and were among the radioactive isotopes released when the Fukushima nuclear reactor was damaged. The Woods Hole Oceanographic Institution has tracked the spread of cesium-134 and -137 in ocean currents from Japan to the western coasts of the United States and Canada. Historically, cesium-137 levels in the Pacific are very low, generally below 2.0 becquerels per cubic meter (Bq / m3). For comparison: The US Environmental Protection Agency (EPA) considers a cesium-137 drinking water level of up to 7,400 Bq / m3 to be safe for human consumption (Figure 1), which is approximately 3,700 times higher than any measured value.
The residents of St. Lawrence Island, located on the northern edge of the Bering Sea, documented the spread of cesium 137 from Fukushima for the first time using water samples.
They collected seawater samples off the Gambell coast for several years, which were then measured by the Woods Hole Oceanographic Institution in Massachusetts. In 2014, 2015, and 2017, the laboratory found very low cesium-137 levels, similar to what happened before the Fukushima nuclear accident. No tests were carried out in 2016 due to a lack of funding.
The cesium-137 content measured in the 2018 seawater sample was 2.4 Bq / m3 and was thus slightly above the values before the accident. Woods Hole scientists attribute this increase to Fukushima-related contamination found in various locations along the west coast of the United States and Canada.
Further information: Radiation from Fukushima, Alaska
Literature
Bundesgesetzblatt Jahrgang 2017 Teil I Nr. 42, – Gesetz zur Neuordnung des Rechts zum Schutz vor der schädlichen Wirkung ionisierender Strahlung vom 27. Juni 2017.
Davis J. J., 1963: Cesium and ists relationships to potassium in ecology. in: Schultz V., Klement A. W. Jr. (eds.): Radioecology. ReinholdPpubl. Comp., New York: 539-556.
Deutscher Bundestag 2007 – Entwurf eines Ersten Gesetzes zur Änderung des Strahlenschutzvorsorgegesetzes.
Hubert, Ph., Perrot, F., Gaye, J., Medina, B., Pravikoff, M. S., 2009: Radioactivity measurements applied
to the dating and authentication of old wines. Comptes Rendus Physique 10, 622-629. Text
IAEA International Atomic Energy Agency, 1991: The international Chernobyl projekt. An overview. Assessment fo radiological consequences and evalution of protective measures. Report by an international advisory committee.
Lederer, M., Hollander, J.M., Perlmann, I., 1967: Table of isotopes. Wiley and Sons. New York.
Katcoff S., 1958: Fission product yields from U, Th, and Pu. Nucleonics 16: 78-85.
Kornberg H. A., 1961: The use of element-pairs in radiation hazard assessment. Health Phys. 6: 46-62.
Meusberger, K., Evrard, O., Alewell, C., Borrelli, P., Cinelli G., Ketterer, M., Mabit, L., Panagos, P., van Oost, K., Ballabio, C., 2020: Plutonium aided reconstruction of caesium atmospheric fallout in European topsoils. Scientific Reports 10, 11858. doi: 10.1038/s41598-020-68736-2
Seelmann-Eggebert W., Pfennig G., Münzel H., 1981: Nuklidkarte. Gesellschaft für Kernforschung mbH. Karlsruhe, 5. Aufl.
Strahlenschutzvorsorgegesetz – Gesetz zum vorsorgenden Schutz der Bevölkerung gegen Strahlenbelastungen(Strahlenschutzvorsorgegesetz-StrVG) ➜ nicht mehr in Kraft
M. De Cort , G. Dubois, Sh. D. Fridman, M.G. Germenchuk, Yu. A. Izrael, A. Janssens, A. R. Jones, G. N. Kelly, E. V. Kvasnikova, I. I. Matveenko, I. M. Nazarov, Yu. M. Pokumeiko, V. A. Sitak, E. D. Stukin, L. Ya. Tabachny, Yu. S. Tsaturov, 1998 and: „Atlas of Caesium Deposition on Europe after the Chernobyl Accident“, EUR report nr. 16733, Office for Official Publications of the European Communities, Luxembourg, Plate 1.
Ziffero M., 1988: A post-chernobyl view. in: Harley J.H., Schnidt G.D., Silini G. (eds.): Radionuclides in the food chain. ILSI Monographs. Springer-Verlag; Berlin, Heidelberg: 3-9.
